Abstract
This article examines the regulatory requirements and scientific basis for Pre-Use Post-Sterilization Integrity Testing (PUPSIT) in pharmaceutical manufacturing. By evaluating the risk-benefit relationship and providing a structured assessment framework, this paper aims to guide manufacturers in making evidence-based decisions regarding PUPSIT implementation. The proposed methodology balances regulatory compliance with patient safety considerations while acknowledging the practical challenges of implementation.
Introduction
Sterile filtration represents a critical control point in the manufacture of parenteral pharmaceutical products. The integrity of sterilizing filters directly impacts product sterility and, consequently, patient safety. European regulatory authorities have increasingly emphasized the implementation of Pre-Use Post-Sterilization Integrity Testing (PUPSIT) as part of a comprehensive filtration validation strategy.
Regulatory Context
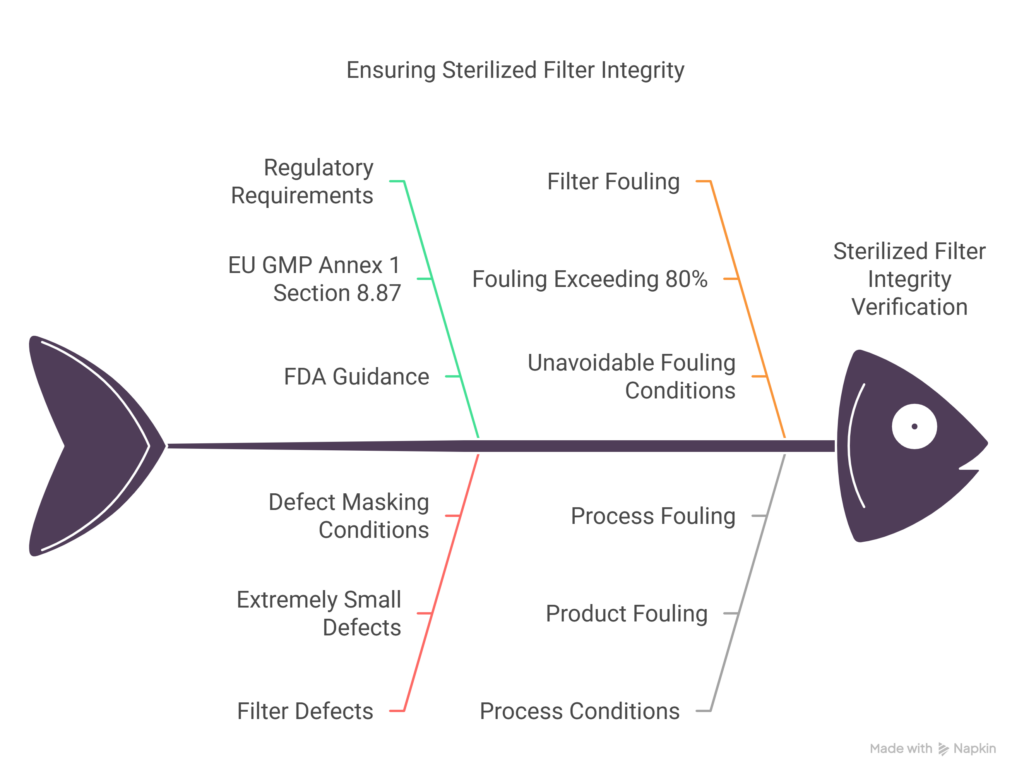
The European Union’s Good Manufacturing Practice (GMP) Annex 1, “Manufacture of Sterile Medicinal Products,” specifically addresses PUPSIT requirements. Section 8.87 of the revised 2022 Annex 1 states that “the integrity of the sterilized filter should be verified by testing before use” (1). This requirement emerges from theoretical concerns that filter defects might be masked during normal processing conditions and subsequently escape detection during post-use testing.
The FDA’s Guidance for Industry on “Sterile Drug Products Produced by Aseptic Processing” similarly acknowledges the importance of filter integrity testing while allowing for risk-based implementation approaches (2).
Scientific Basis and Risk Considerations
The primary regulatory concern regarding filter integrity involves the potential for defect masking. Research indicates that such occurrences are rare and typically occur only under specific conditions. Studies have demonstrated that defect masking primarily occurs when:
- The filter defect is extremely small
- Filter fouling exceeds approximately 80%
- Product characteristics and processing conditions create circumstances where such fouling cannot be mitigated (3)
Despite the limited probability of occurrence, these scenarios warrant careful consideration within a comprehensive risk management framework.
Risk Assessment Methodology
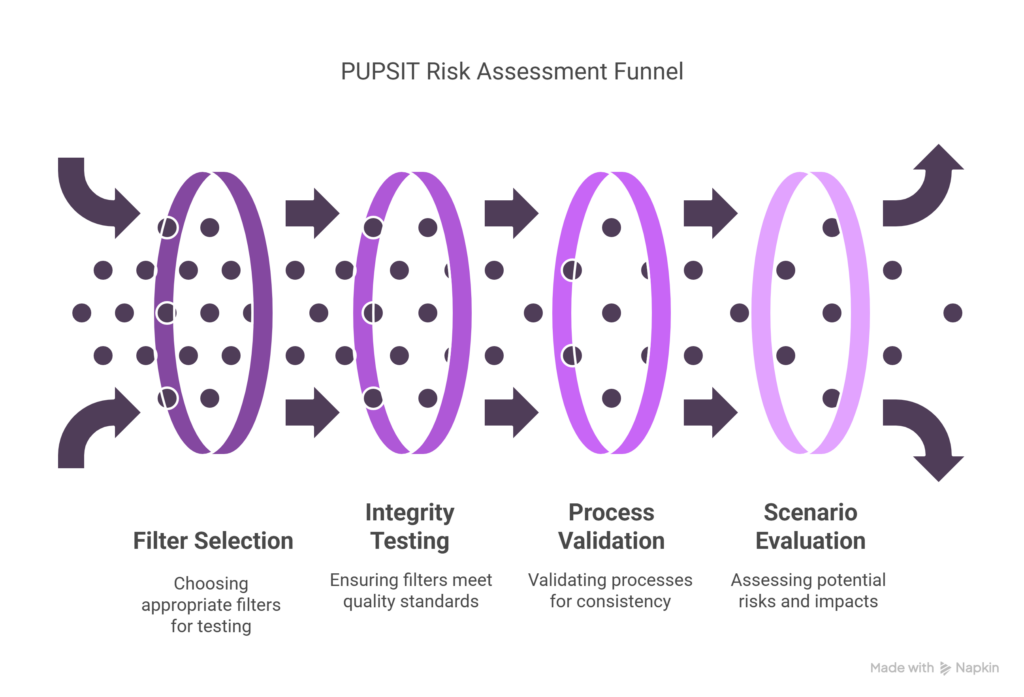
Before implementing PUPSIT, manufacturers should conduct a thorough risk assessment of their filtration process design, including:
- Filter selection and qualification
- Integrity testing methods and parameters
- Process validation strategies
- Process-specific considerations
A structured risk assessment should evaluate multiple scenarios, including:
- Filter damage during handling or sterilization
- Product characteristics that may affect filter integrity
- Processing conditions that might contribute to defect masking
- Detection capabilities of current integrity testing methods
- Potential impacts on product quality and patient safety
Implementation Considerations
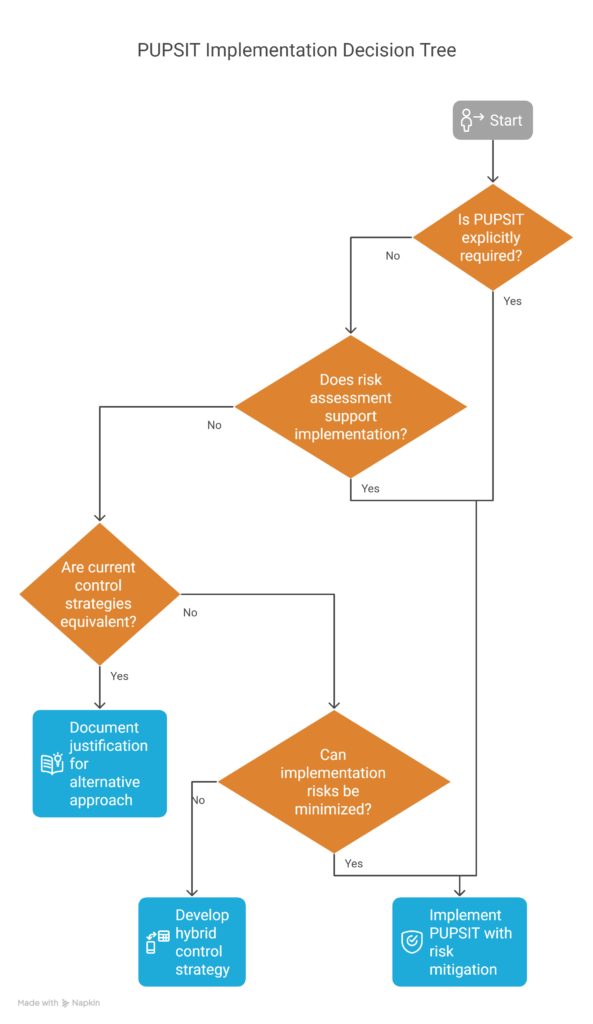
Where PUPSIT implementation is not explicitly mandated, manufacturers should provide robust evidence demonstrating either:
- That the risk-benefit analysis supports PUPSIT implementation, with associated risks minimized to the extent possible, or
- That current control strategies provide equivalent assurance of filter integrity without PUPSIT implementation
These assessments should focus on patient safety implications rather than procedural compliance alone. The PDA Technical Report No. 79 provides valuable guidance for developing risk-based approaches to filter integrity testing strategies (4).
Standardized Approach
The risk assessment framework proposed herein offers a standardized methodology benefiting both industry and regulators by:
- Establishing consistent evaluation criteria
- Prioritizing patient-centric outcomes
- Supporting evidence-based decision-making
- Facilitating transparent regulatory communication
This approach enables organizations to implement appropriate control strategies aligned with both regulatory expectations and patient safety priorities.
Conclusion
Rather than adopting universal implementation policies, a risk-based approach to PUPSIT allows manufacturers to make informed decisions prioritizing product quality and patient safety. By systematically evaluating potential failure modes and their consequences, organizations can develop integrity testing strategies that effectively mitigate risks while acknowledging practical implementation considerations.
The decision to implement PUPSIT should ultimately rest on a comprehensive assessment of whether such testing enhances patient protection within specific manufacturing contexts. This evidence-based approach aligns regulatory compliance with meaningful quality outcomes, serving the interests of patients, manufacturers, and regulatory authorities alike.
References
- European Commission. (2022). EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use, Annex 1: Manufacture of Sterile Medicinal Products.
- Food and Drug Administration. (2004). Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice.
- Parenteral Drug Association. (2018). Technical Report No. 26 (Revised 2008): Sterilizing Filtration of Liquids.
- Parenteral Drug Association. (2019). Technical Report No. 79: Particulate Matter Control in Difficult to Inspect Parenterals.
