PRES Community of Practice (CoP)
Vision Statement:
Learn, Share the knowledge and Let’s Grow Together
About PRES CoP
Since 2012 we are providing the technical solution with USCGMP platform. PRES is created to provide enhance experience and real time connectivity for technical solution. Our WhatsApp group exist from 2015, earlier it was named as EU Annex. 15.
Pharmaceutical resource and education services (PRES) , it’s a profit free organization
PRES CoP aims to communicate with industry folks in a real time to discuss and provide best possible technical solutions.
Our aim to make connection with the people those who are handling sterile product manufacturing, discuss , exchange information and share learning.
Who Should Join This Group ?
Anyone from the industry working with sterile drug manufacturing.
And Willing to contribute personal time for self development , learning , sharing knowledge and networking for better career growth.
How we operate ?
We discuss problems and try to provide solution as group on WhatsApp Platform.
We are committed for monthly meeting or Planned Group call which need deeper discussion apart from the WhatsApp platform.
Making a inventory which will provide direct access to copyright free regulatory Guidance document. https://1drv.ms/f/s!Ag62rKN9sLcvgi6sfareDuMtN7n6?e=oBgmSZ
Deliver scientific write up which can be use as common inventory between industry. And publish
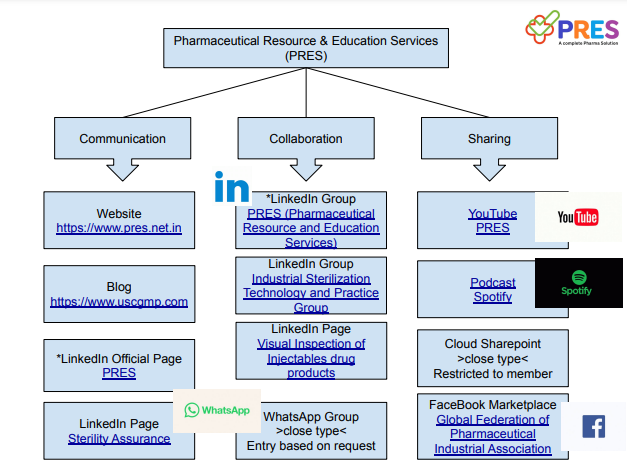
Useful links,
PRES Website : https://www.pres.net.in
Blog :https://www.uscgmp.com
YouTube Channel, https://www.youtube.com/@cGMP
PODCAST link, https://podcasters.spotify.com/pod/show/palash-chandra-das
Email : palash.ds@gmail.com and mail2uscgmp@gmail.com
LinkedIn page , https://www.linkedin.com/company/pharmaceutical-resource-and-education-services/
Download the details PDF with link >>>>>
Subject Matter Expert available for following area : <Wanted to nominate as SME , Apply With the link>
Sterile Injectable Manufacturing
- Primary mentor for aseptic processing
- Primary mentor for formulation
- Primary mentor for fill/finish operations
Validation and Qualification
- Primary mentor for utility systems qualification (HVAC, water, gases)
- Primary mentor for equipment/machine qualification
- Primary mentor for instrument qualification
- Primary mentor for process validation
Quality Systems
- Primary mentor for investigations
- Primary mentor for CAPA implementation
- Primary mentor for change management
Regulatory Affairs
- Primary mentor for regulatory submissions
- Primary mentor for inspection readiness
- Primary mentor for regulatory intelligence/updates